How Many Different D Orbitals Are Within the 3d Sublevel
The first energy level contains only one s orbital the second energy level contains one s orbital and three p orbitals and the third energy level contains one s orbital three p orbitals and five d orbitals. The five 3d orbitals are called.
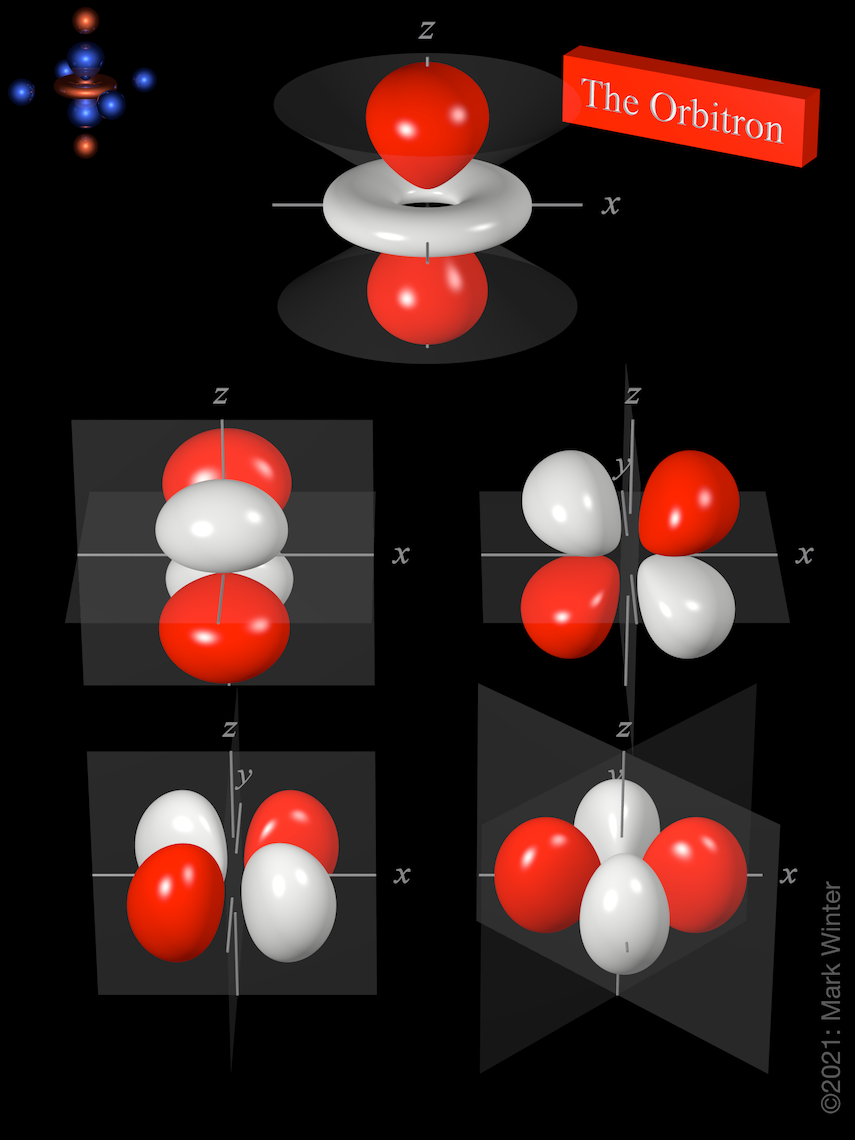
The Orbitron 3d Atomic Orbitals
By admin July 16 2021.

. Each orbital can hold up to two electrons meaning that the 1s 2s 3s 4s and 5s can hold two electrons. 1 to observe the. Within the n4 l2 sublevel the electron moves from m_l-1 to m_l1.
In an atom dropping from one energy level to a lower one. Specifically this project had two aims. Specifies which orbital within a sublevel you are likely to find the electron.
It is 26 editions old. This quantum number has integral values from 0 up to n-1. It determines the orientation of the orbital.
Atomic emission spectra are due to electrons. 2p and 3p orbitals Check all that apply. Preface The Essentials of Physical Chemistry has been written for BSc students.
Which of the following statements correctly reflects the most important consequence of Paulis exclusion principle. To calculate electron shell capability you first need to determine the number of electrons possible per shell. In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels.
Assisting students with assignments online. At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz. The Journal of Chemical Physics 38 2686 1963.
Ionization energy of atoms denoted E i is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound atomic electronsThe measurement is performed in the gas phase on single atoms. 5s and 6s orbitals Check all that apply. 33p and 4d sublevels Check all that.
An atomic orbital can hold a maximum of two. Read Doc 117 b p s xi chemistry iit jee advanced study package 2014 15 by SDharmaraj on Issuu and browse thousands of other publications on our p. View Answer Fill in the blank.
View Answer List the quantum numbers of all of the electrons in a. The shape not the size of an electron cloud is determined by the electrons ____. At the third level there are a total of nine orbitals altogether.
It has been used by more than 2 million students. Each ℓ is a different orbital shape or orbital type. There are many energy levels within the H atom.
To the s p d f in order of increasing energy. There are several other particles within the H atom. For a many-electron atom which of the following sublevels has the highest energy.
Magnetic quantum number mℓ-2 -1 0 1 2. The d type has five different orbitals. The 2p3p 4p and 5p can each hold six electrons because they have three orbitals.
It has been national bestseller for more than 65 years. While only noble gases occur as monatomic gases other gases can be split into single atoms. Because of the effects of shielding and the different radial distributions of orbitals with the same value of n but different values of l the different subshells are not degenerate in a.
1072011 91823 PM Answer each question with as many details as possible from the novel. Data from E. Get 247 customer support help when you place a homework help service order with us.
The f type has seven orbitals. The electron _____ of an element shows the distribution of electrons within the electronic energy levels of the atom. Elizabeth Bogal-Allbritten Created Date.
It really has been that long. Within each energy level the s orbital is at a lower energy. What is the maximum number of d orbitals in a principal energy level.
There are several different orbital shapess p d and fbut we will be focusing mainly on s and p orbitals for now. The ground state energy of the hydrogen atom is -136 eV. How many orbitals in an atom can have the designation 5p 3d_z2 4d n 5 n 4.
Name the type of conflict that takes place within a characters mind. To pass the quiz youll need to. Different lines are generated by different H atoms.
How many energy sublevels are in the second principal energy level.
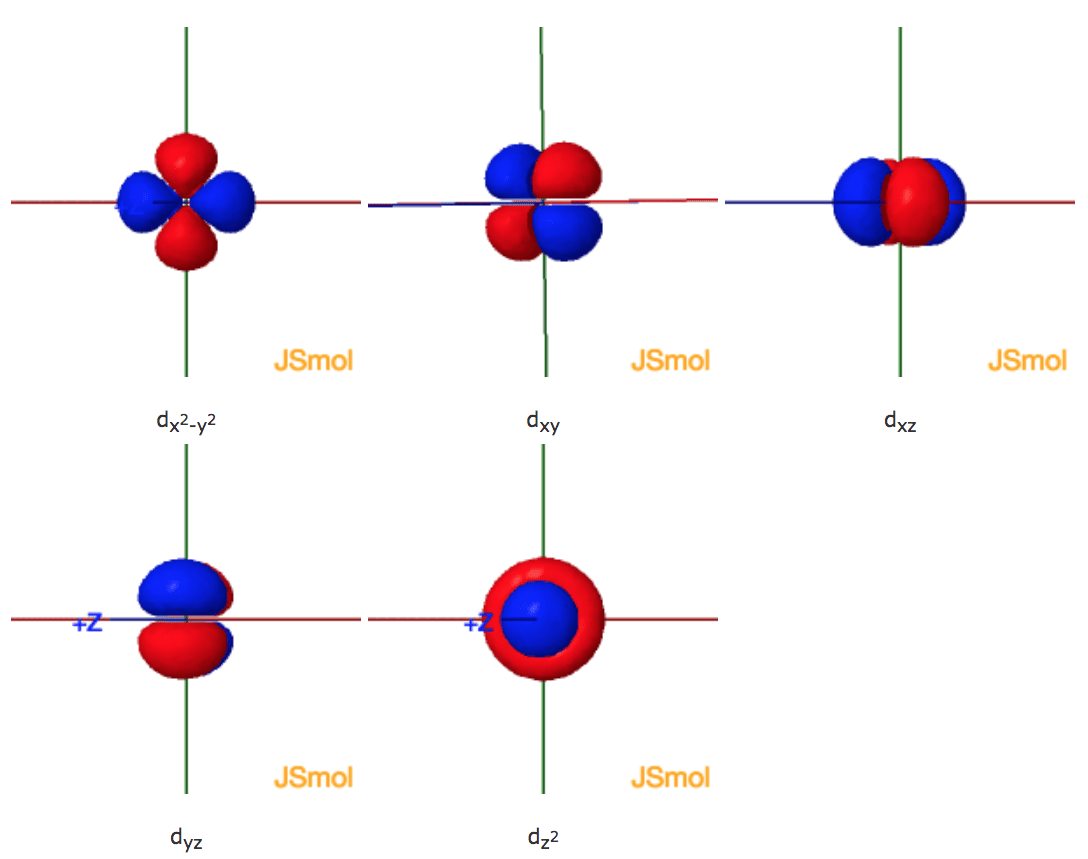
Shapes Of The 3d Orbitals In 3d
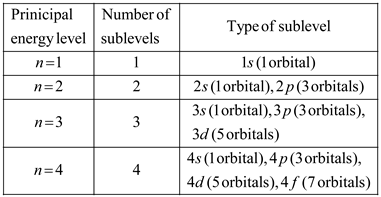
Solved Chapter 5 Problem 7sa Solution Prentice Hall Chemistry Student Edition 2008c 1st Edition Chegg Com
No comments for "How Many Different D Orbitals Are Within the 3d Sublevel"
Post a Comment